Calcium Chloride and Hydrochloric Acid Balanced Equation
The balancing of a chemical equation is based on the law of conservation of mass. P ΔH ΔQ ΔV.

How To Balance Ca Hcl Cacl2 H2 Calcium Hydrochloric Acid Youtube
You want to measure how much water is produced when 120 g of glucose C_6H_12O_6 is burned with enough oxygen.

. What volume of 345 M calcium chloride solution can be prepared using all of 167 grams of calcium. Click hereto get an answer to your question Metal compound A reacts with dilute hydrochloric acid to produce effervescence. In chemistry acid hydrolysis is a process in which a protic acid is used to catalyze the cleavage of a chemical bond via a nucleophile substitution reaction with the addition of the elements of water H 2 O.
It slightly dissolves in water. An acid is a substance which produces hydrogen ions H aq in water. It is a strong corrosive acid with a chemical formula HCl.
To learn more about the Preparations Properties Uses and Videos with FAQs of. Only coefficients should be added or changed when balancing chemical equations. Yield actual yieldtheoretical yield 100 So lets say you want to do an experiment in the lab.
Metal compound A reacts with dilute hydrochloric acid to produce effervescence. Yes this can be done through a chemical equation. Milk of magnesia chemically magnesium hydroxide is used as an antacid.
The gas evolved extinguishes a burning candle. Concentrated H 2 SO 4. Percent yield represents the ratio between what is experimentally obtained and what is theoretically calculated multiplied by 100.
Please resubmit the question and. The Ca2 and the NO3 ions remain in solution and are not part of the reaction. HCl Acid Hydrochloric Acid - Hydrochloric acid is an inorganic chemical.
Write a balanced chemical equation for the reaction if one of the compounds formed is calcium chloride. A chemical equation can be made more concise and useful if we use chemical formulae instead of words. The acidity produced due to excess hydrochloric acid in the stomach which cause indigestion produce pain and irritation.
The gas evolved extinguishes a burning candle. For example the ionic equation for the double displacement reaction of Calcium Chloride with Silver Nitrate in an aqueous solution would be. Ca2 2Cl 2Ag 2NO3 Ca2 2NO3 2AgCls In the above mentioned reaction only Ag2 ions and Cl- ions take part in the reaction.
It is also known as hydrogen chloride or muriatic acid. Aluminum and hydrochloric acid react to form aluminum. Write the ionic equation for the word equation.
22 WHAT DO ALL ACIDS AND ALL BASES HAVE IN COMMON. Phosphorous pentoxide and calcium oxide are good drying agents but they cannot be used to dry hydrogen chloride gas because they react with hydrogen chloride. A coefficient is a number to the left of a formula and it applies to the entire formula it precedes.
Since it is basic in nature reacts with the excess hydrochloric acid present in the stomach and neutralises it. The balanced equation for the reaction. Sodium chlorideaq silver nitrateaq silver chlorides sodium nitrateaq Solution.
Definition of An Acid For example hydrochloric acid dissolves in water to form hydrogen ions and chloride ions. This kind of unbalanced equation is called a skeletal chemical equation. Therefore the reaction between calcium carbonate and hydrochloric acid is.
Speight in Reaction Mechanisms in Environmental Engineering 2018 31 Acid Hydrolysis. Chapter 10 Acids Bases and Salts. Since we only answer up to 3 sub-parts well answer the first 3.
Na2SO4 BaCl2 question_answer Q. Consider the balanced reaction of sodium sulfate and barium chloride is as follows. Calcium phosphate appears as a white amorphous or crystalline powder that is odourless and tasteless.
In Section 21 we have seen that all acids have similar chemical. When the total number of atoms of each element is equal on both sides of the equation then the equation is known as a balanced chemical equation. Write the equation and balance it if necessary NaClaq AgNO 3 aq AgCls NaNO 3 aq Step 2.
P 7 41 11. In the context of a balanced chemical equation a subscript is a number to the lower right of an element or ion within formula and it applies to only the part of the formula it follows. Write a balanced chemical equation for the reaction if one of the compounds formed is calcium chloride.
As the end product is calcium chloride and the gas formed is carbon dioxide the metal compound A must be calcium carbonate. Only compounds that are aqueous are split into ions. HClaq H aq Cl- aq It is the hydrogen ions which turn blue litmus to red and give acids their characteristic properties.
How can I balance this chemical equations. Calcium Chlorine Calcium chloride 412 Writing a Chemical Equation Is there any other shorter way for representing a chemical change. In the previous lesson you.
The drying agent used in drying hydrogen chloride gas is conc. However an online Chemical Equation Balancer Calculator will provide you the balanced equation equilibrium constant with chemical name and formula of all reactants and product of a chemical equation. It is insoluble in ethanol and acetic acid but soluble in dilute nitric acid and hydrochloric acid.
A 00665 g sample of aluminum metal reacts with hydrochloric acid to give 905 mL of hydrogen gas at A. Write a balanced chemical equation for the reaction if one of the compounds formed is calcium chloride. By making considerable change in enthalpy equation we get.
It is found in. A common type of hydrolysis occurs when a salt of a weak acid or. According to the.

Hcl Ca Oh 2 Cacl2 H2o Chemical Equation Balancer
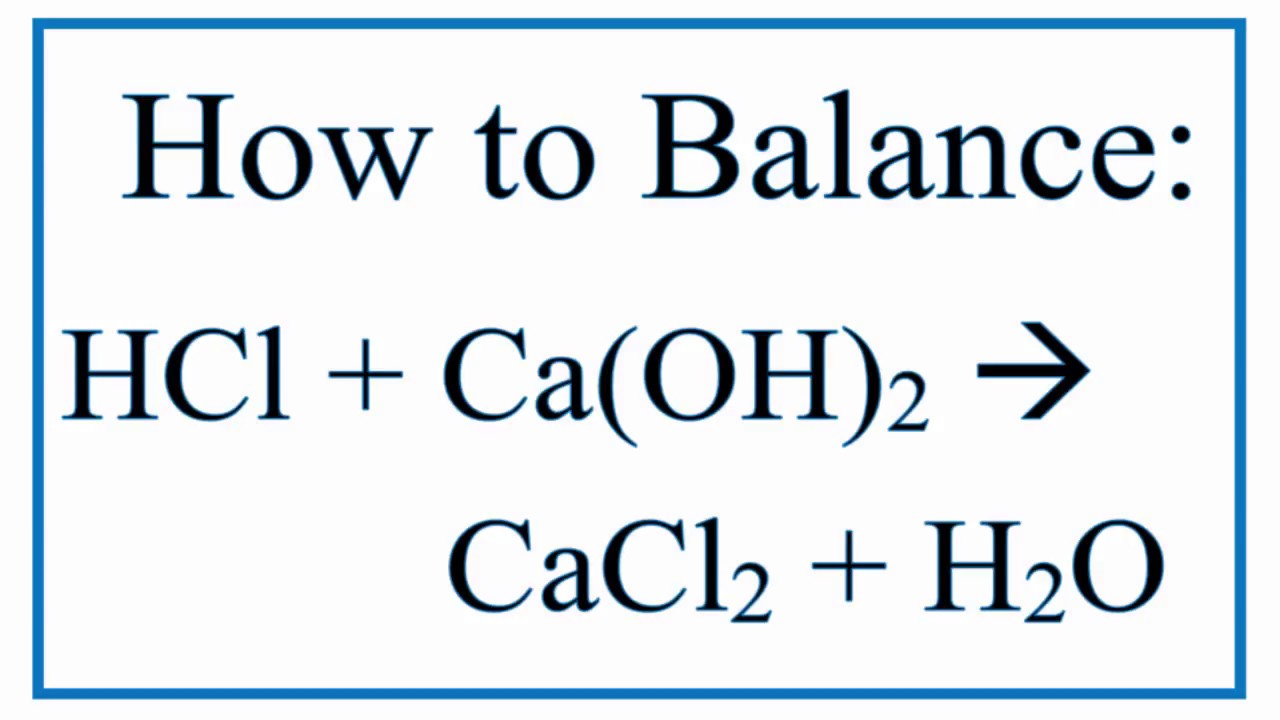
Balance Hcl Ca Oh 2 Cacl2 H2o Hydrochloric Acid And Calcium Hydroxide Youtube

How To Balance Caco3 Hcl Cacl2 Co2 H2o Calcium Carbonate Hydrochloric Acid Youtube

Comments
Post a Comment